Pipe Cleaner and Pony Bead Covalent Molecules
We took a quiz in physical science on Monday over predicting the products of a reaction. Since Thanksgiving Break started Wednesday, I definitely did not want to start a new section on Tuesday. I had seen an idea on twitter the week AFTER we finished discussing ionic vs. covalent compounds. I decided to save the idea for next year until, of course, I realized we had an extra class day before Thanksgiving Break.
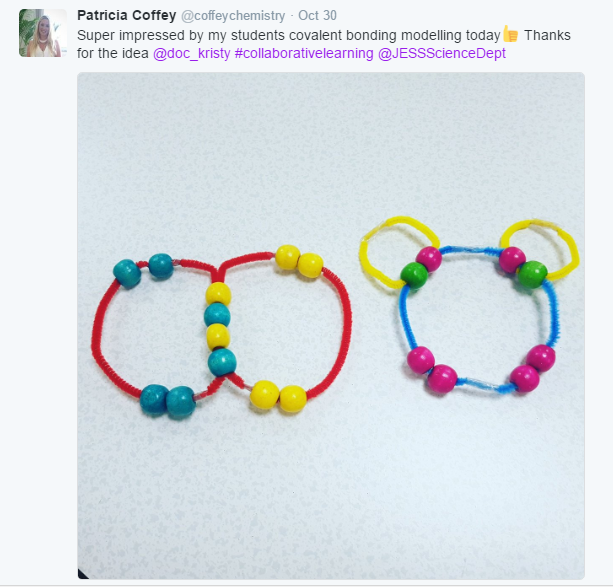
It didn’t end up going exactly as planned…

After setting up the supplies and demonstrating to students how to build a water molecule, the intercom buzzed. All freshmen were to report to the library immediately for a class meeting. Have I mentioned that physical science is intended to be a freshmen level class? This meant I was left with two students in my classroom for the rest of the class period.
It’s okay. They each built their own molecule, and we pinned them to the bulletin board anyway. I guess it was a good thing I hadn’t planned anything new for that class period!
Here are our finished products:

My water molecule:

A students’ ammonia molecule:

And, another student’s methane molecule:

I’m sad I couldn’t do these with my entire class, but that’s just the reality of the day before Thanksgiving Break. I do want to incorporate these into our unit on covalent compounds in the future!


Any modifications you'd do for a middle school classroom?