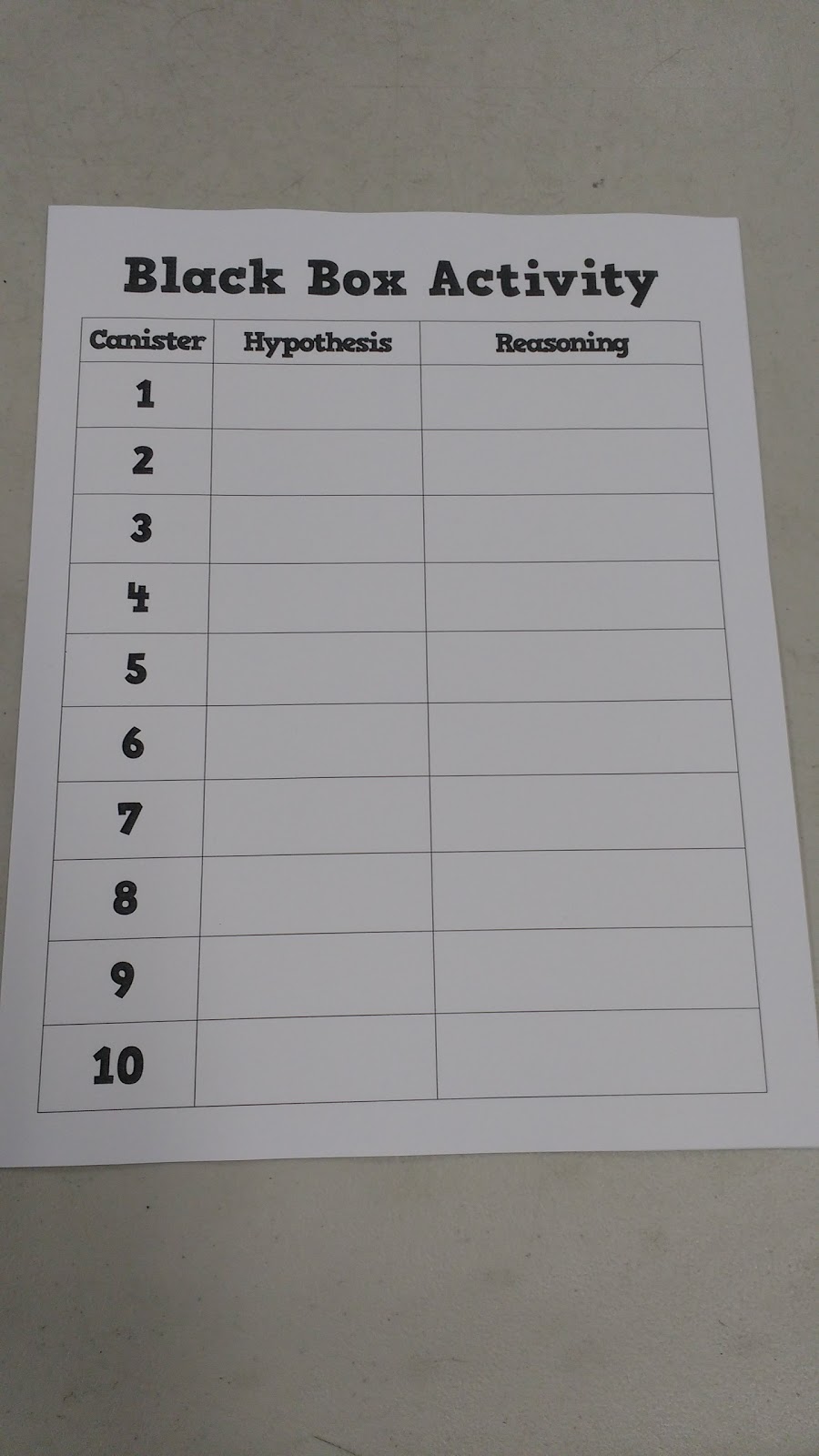
Chemistry Black Box Activity
This blog post contains Amazon affiliate links. As an Amazon Associate, I earn a small commission from qualifying purchases.
Earlier this week, I challenged my chemistry students to a Black Box Activity Challenge. The idea is that they would be given containers where they could not see the contents. They would have to use their resources to create a hypothesis regarding what was in the container. My goal was to give my students a taste of what it was like for early scientists doing atomic research without being able to see the atoms they were theorizing about.

My students worked in five groups. Each group received a Black Box Activity Recording Sheet where they listed their hypothesis and their reasoning.
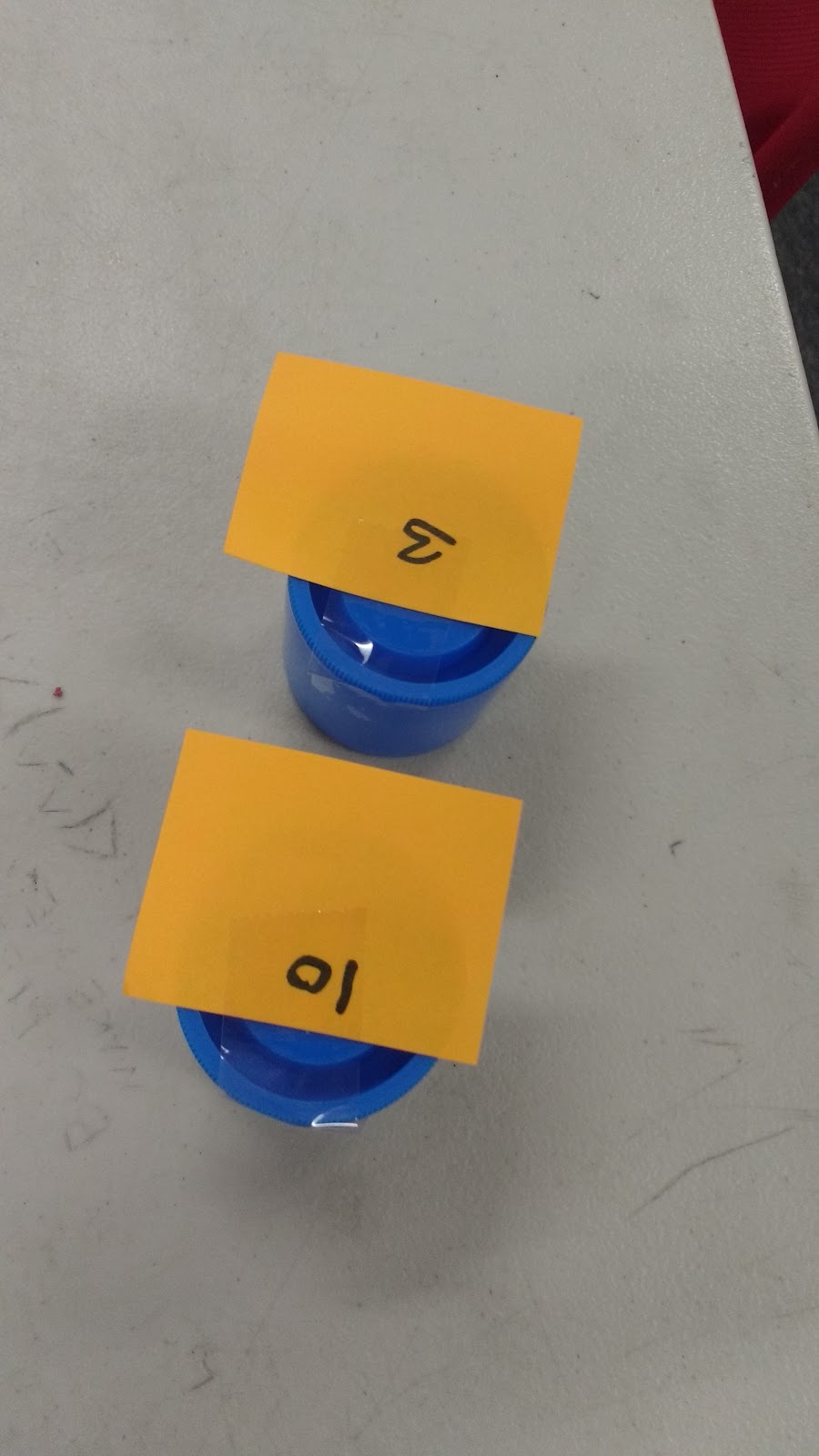
I didn’t have any literal black boxes sitting around, so I decided to use the film canisters that I purchased early this year for our density challenge lab.

My husband helped me to open the film canisters and pack them with goodies to be hypothesized about.
We filled 10 different canisters for this activity. Here’s a peek inside a few of them.
Air. Or, as my students declared, “Empty!”

Tile Spacers. This one threw all of my students for a loop. It turned out that none of them had ever heard of tile spacers before. I purchased these tile spacers to make a positive/negative game for my math concepts class. I’ve never got around to making the game, though… Maybe I’ll have to add it to my summer project list!

Bingo Chips. One group mistook these for coins.

Foam Cube. I purchased these foam cubes from Dollar Tree a few years ago to turn into dice. I only used a few, so I just have them sitting in my foam dice tub doing nothing. I loved hearing kids debate about what material the cube was made of after they decided it must be a cube.

Cotton Ball. Several groups thought this was a crumpled piece of paper instead.

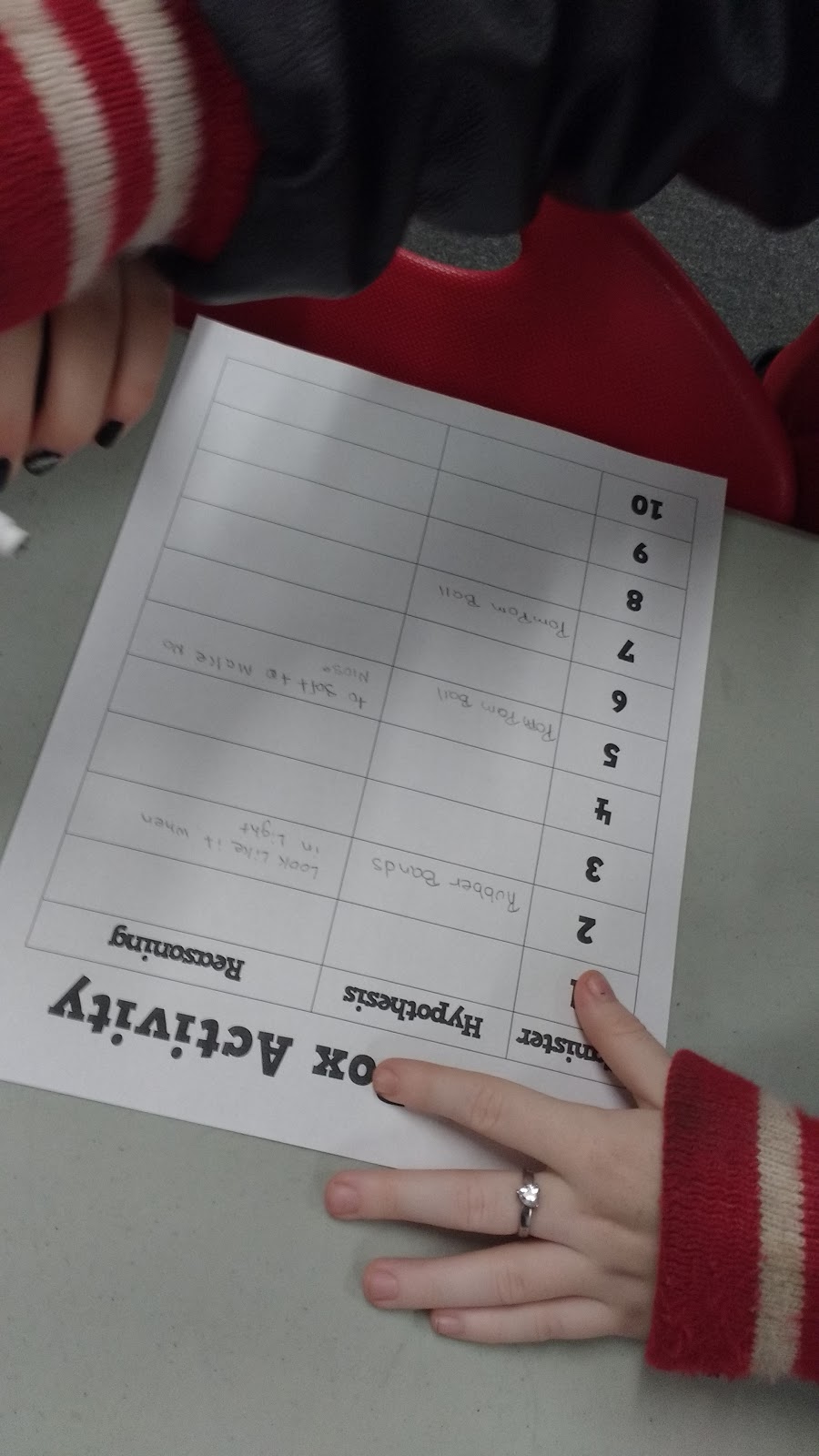
Pom Poms. One group called these fuzz balls. Another group thought this canister held pink and green slime.

Rubberbands.

Not pictured were a marble, two dice, and some paperclips.
I stuck a post-it note to each canister with a number on it to keep my students’ guesses organized. The post-it notes didn’t stick to the film canisters very well, so I ended up taping them on.
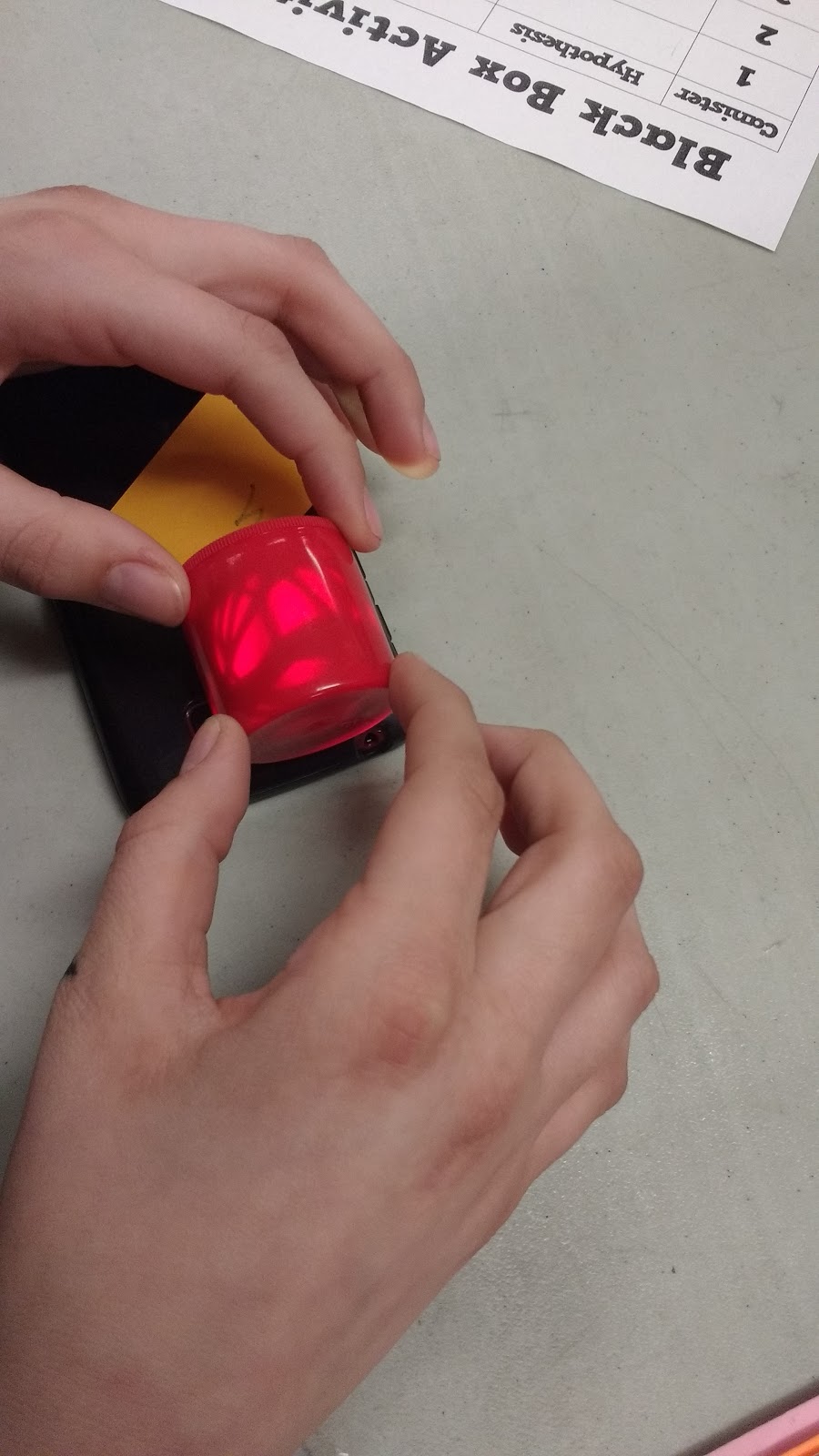
I told students that they could use any resource in the classroom to create their hypothesis. The only thing they couldn’t do was open the container.

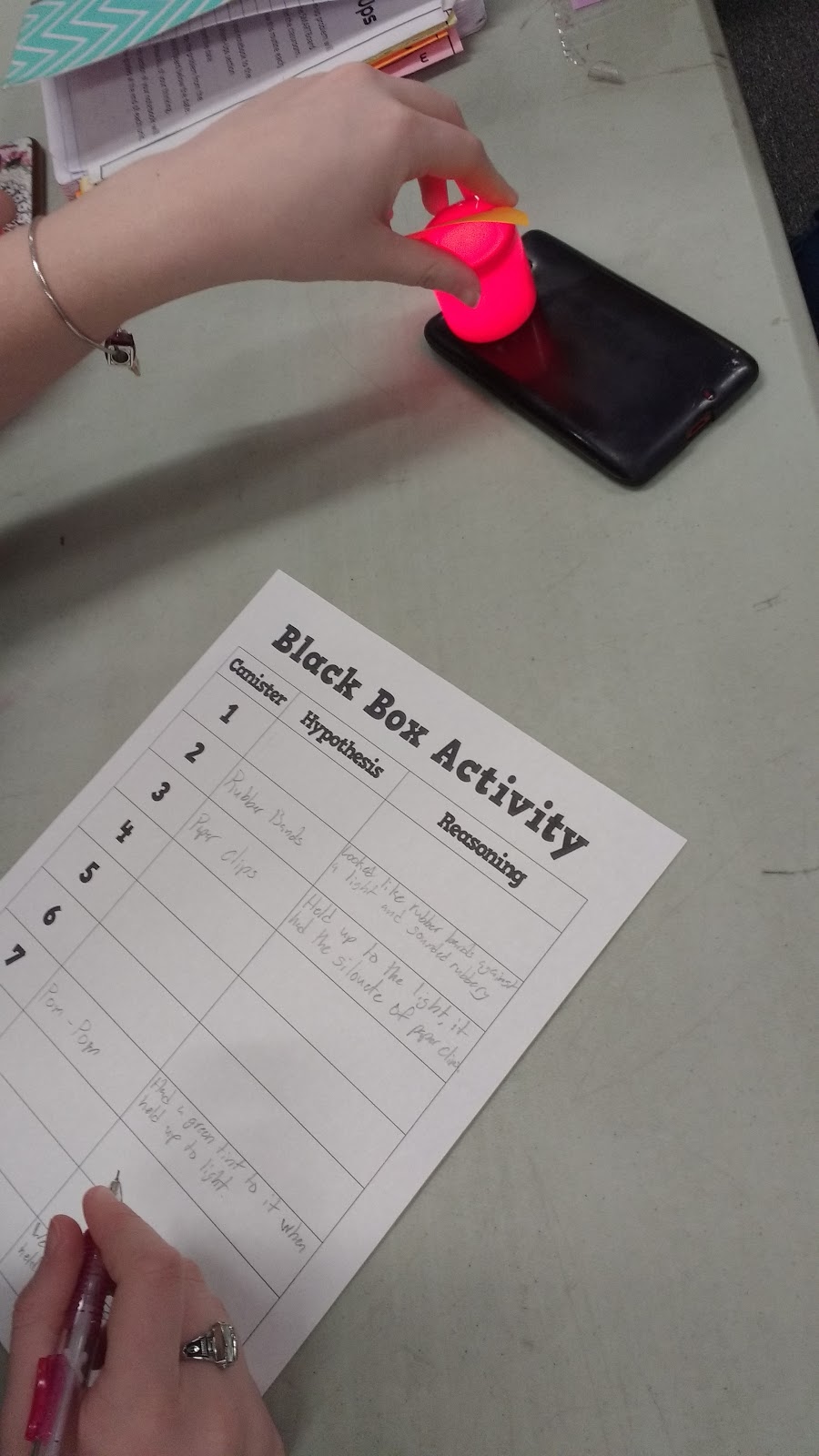
And, this is how I learned that red and blue film canisters are not truly opaque. They become quite translucent when you place them next to a cell phone flash light.

Note to Self: Invest in a set of black film canisters for next time. Look at how easily you can see the rubberbands below!
Even with the help of their cell phone lights, they did still have to do some hypothesizing. The light gave them an idea of what was inside, but they had to apply what they were seeing to previous experiences to write their hypothesis.
My students really enjoyed this activity, and they were DYING to know what was in the canisters.
After all of the groups were finished, we did a reveal of the contents of each film canister. Each group shared what they thought was in canister 1. Then, we opened canister 1. None of the groups got all 10 correct thanks to those pesky tile spacers!
Files for Chemistry Black Box Activity
Click here to SAVE the file to your device.
Black Box Activity (PDF)
2021 saves – 220.19 KB
Please check out my other chemistry resources for more free activities.




I've done activities like this with probability where students draw marbles out of a bag one at a time (replacing them each time) and record the results after 10, 25, and 50 pulls. They then make a prediction about the contents of the bag – how many of each colored marble are in the bag? The twist at the end is that I don't reveal the total contents of the bags at the end! Why not? Well, as I tell the students, they were determining the contents based on a sample.
Scientists have to do that every day! They take blood samples and use that one sample to determine what types of medicine to prescribe or what conditions their patients may or may not have. They don't have the luxury of analyzing every drop of a patient's blood. Plus, at the "end" no one is there to tell them, "Yes, that's exactly what was in there!" It's a rough analogy, but really drives home the concept. Another example is cooking – chefs can sample a spoonful of soup to see if it needs more seasoning, but eating the entire pot would not really make sense! 🙂