Effect of Concentration on Reaction Rate Lab
When we got back from Christmas Break, I realized that we still hadn’t done any labs to explore the effect of concentration on reaction rates. Frustrated from how our previous labs had gone, I decided to just do a whole-class demonstration so we could move on to the next skill.
We worked together to create three different concentrations of water and vinegar in three graduated cylinders. We measured the same amount of baking soda into three balloons that would be placed over the graduated cylinders to produce a closed system.
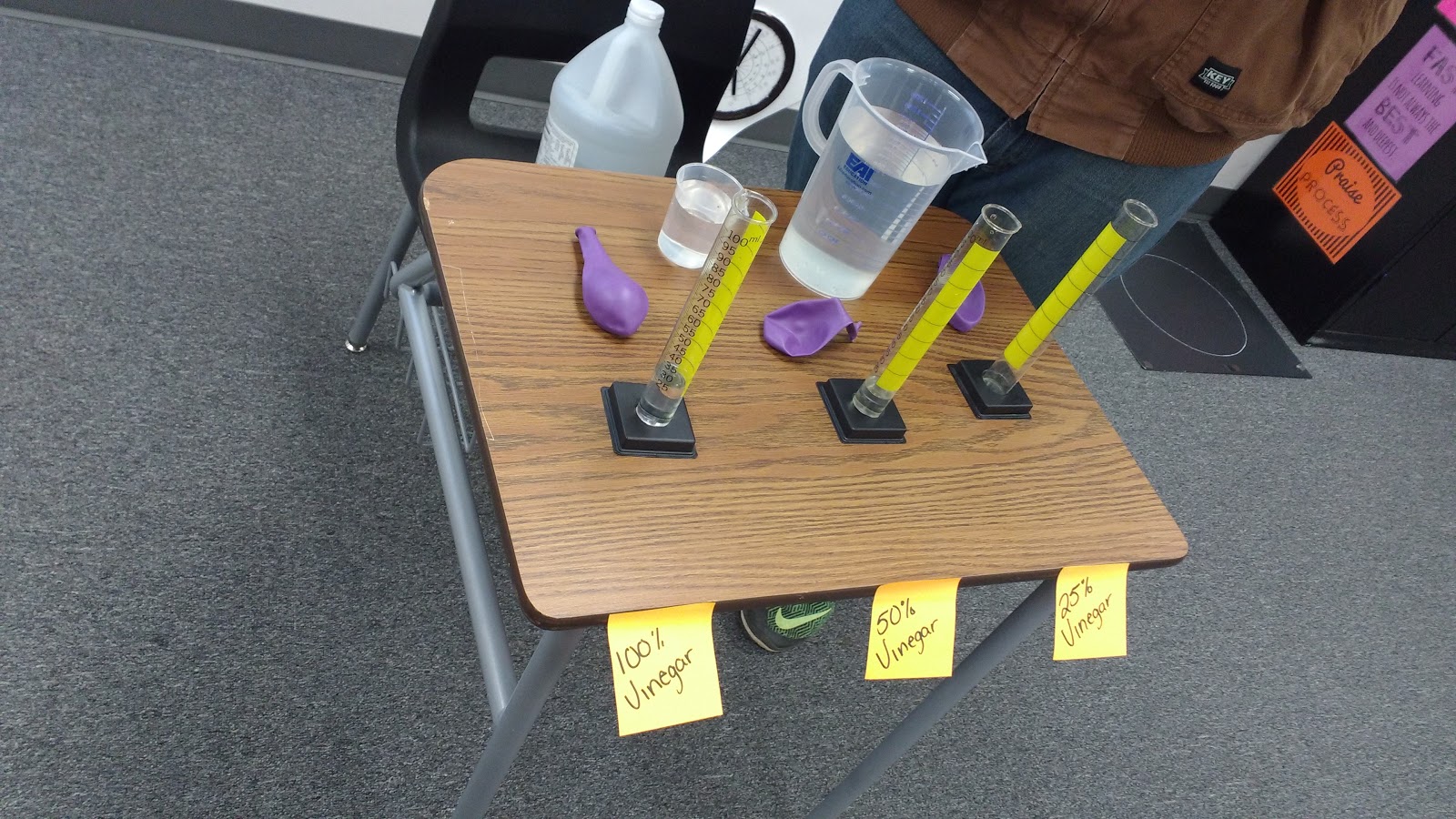
Three students stood behind the graduated cylinders and lifted up the balloons at the exact same time to release the baking soda into the vinegar/water mixtures. Of course, we made lots of predictions first.

Here’s a video of our reactions taking place. If the embedded video doesn’t show up below, here is a direct link to the video. You can hear how excited my students were!